According to the Bronsted-Lowry definition,
an acid is a proton acceptor
a b...

Chemistry, 19.03.2020 21:01 gokusupersaiyan12345
According to the Bronsted-Lowry definition,
an acid is a proton acceptor
a base is a proton donor
a base produces H+ ions in aqueous solutions
a base is a proton acceptor

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 15:00
Answer explain why it is not possible to deduce a complete order of reactivity.
Answers: 3

Chemistry, 22.06.2019 21:00
Write a balanced equation showing the formation of copper (ii) nitrite from its elements
Answers: 1

Chemistry, 23.06.2019 15:30
How many moles of potassium nitrate, kno3 are present in a sample with a mass of 85.2 g?
Answers: 1
You know the right answer?
Questions

Physics, 21.07.2019 16:00

Biology, 21.07.2019 16:00

Mathematics, 21.07.2019 16:00


History, 21.07.2019 16:00


History, 21.07.2019 16:00

Physics, 21.07.2019 16:00


Mathematics, 21.07.2019 16:00



History, 21.07.2019 16:00

English, 21.07.2019 16:00


Mathematics, 21.07.2019 16:00

English, 21.07.2019 16:00

Spanish, 21.07.2019 16:00


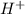 + Conjugate base
+ Conjugate base


