
Determine the units of the rate constant for all four reactions listed in the problem above, and enter the correct choices from the list below. Enter 4 letters in order (e. g. ABCD or CBED)a. mole L-1 sec-1
b. mole-1 L sec-1
c. mole2 L-2 sec-1
d. mole-2 L2 sec-1
e. None of the above.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

Chemistry, 22.06.2019 15:20
Water is initially present in a state where its molecules are far apart. during a change of state, its molecules slow down. which change of state has most likely taken place? from a gas to a liquid from a liquid to a gas from a solid to a liquid from a gas to a plasma
Answers: 1

Chemistry, 22.06.2019 17:20
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2
You know the right answer?
Determine the units of the rate constant for all four reactions listed in the problem above, and ent...
Questions

Mathematics, 28.05.2021 03:20

Mathematics, 28.05.2021 03:20

Mathematics, 28.05.2021 03:20


Physics, 28.05.2021 03:20

Biology, 28.05.2021 03:20



Computers and Technology, 28.05.2021 03:20

Computers and Technology, 28.05.2021 03:20



Mathematics, 28.05.2021 03:20

Geography, 28.05.2021 03:20






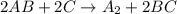 , the following slow first steps have been proposed.
, the following slow first steps have been proposed.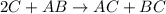
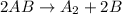
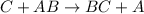
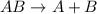
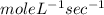
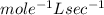
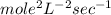
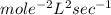
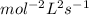
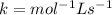
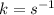
![Rate=k[C]^2[AB}^1](/tpl/images/0554/4588/ed857.png)
![molL^{-1}s^{-1}=k[molL^{-1}]^2[molL^{-1}}^1](/tpl/images/0554/4588/5c165.png)
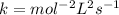
![Rate=k[AB]^2](/tpl/images/0554/4588/428db.png)
![molL^{-1}s^{-1}=k[molL^{-1}]^2](/tpl/images/0554/4588/5d3a7.png)
![Rate=k[C}^1[AB]^1](/tpl/images/0554/4588/c1ce9.png)
![molL^{-1}s^{-1}=k[molL^{-1}]^1[molL^{-1}]^1](/tpl/images/0554/4588/98d2a.png)
![Rate=k[AB]^1](/tpl/images/0554/4588/ca45b.png)
![molL^{-1}s^{-1}=k[molL^{-1}]^1](/tpl/images/0554/4588/dfb84.png)


