
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The first step is a fast reversible step and the second is a slow reaction between an oxygen atom and an ozone molecule:Step 1: O3(g) O2(g) + O(g) Fast, reversible, reactionStep 2: O3(g) + O(g) → 2O2(g) SlowWhich is the rate determining step?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If a bottle of olive oil contains 1.4 kg of olive oil, what is the volume, in milliliters ( ml ), of the olive oil?
Answers: 1

Chemistry, 21.06.2019 20:40
Astudent made the lewis dot diagram of a compound shown. what is the error in the lewis dot diagram? a)an o atom should transfer all of its six electrons to mg because the formula is mgo b) both electrons of mg should be transferred to one o adam because the formula is mgo c) the electrons should be transferred from each o add him to capital mg because mg has fewer electrons d) the number of dots around mg should be four because it has to transfer two electrons to each o
Answers: 1

Chemistry, 22.06.2019 11:50
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3
You know the right answer?
The decomposition of ozone in the upper atmosphere to dioxygen occurs by a two-step mechanism. The f...
Questions

History, 10.12.2020 17:30


Mathematics, 10.12.2020 17:30

Arts, 10.12.2020 17:30


Mathematics, 10.12.2020 17:30

Mathematics, 10.12.2020 17:30


Mathematics, 10.12.2020 17:30



Biology, 10.12.2020 17:30


Social Studies, 10.12.2020 17:30





Mathematics, 10.12.2020 17:30

Mathematics, 10.12.2020 17:30

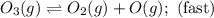
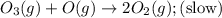
![\text{Rate}=k[O_3][O]](/tpl/images/0554/3771/ba34f.png)


