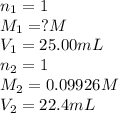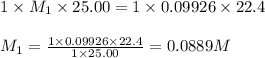Chemistry, 19.03.2020 18:34 wafflewarriormg
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and the equivalence point volume was determined by graphical means to be 22.4 mL. What is the concentration of the acetic acid?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 13:20
Can someone me with 3 and 4 plz. this is for masteries test.
Answers: 2

Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2

Chemistry, 22.06.2019 21:30
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
You know the right answer?
A 25.0 mL of a solution of acetic acid of unknown concentration is titrated with 0.09926 M NaOH and...
Questions




Mathematics, 30.01.2021 02:00

Geography, 30.01.2021 02:00

Social Studies, 30.01.2021 02:00


Mathematics, 30.01.2021 02:00





Mathematics, 30.01.2021 02:00



English, 30.01.2021 02:00

History, 30.01.2021 02:00

Social Studies, 30.01.2021 02:00


English, 30.01.2021 02:00

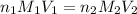
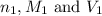 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 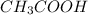
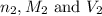 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.