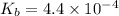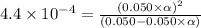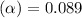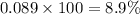Chemistry, 19.03.2020 18:21 colton6926
Consider the chemical equation for the ionization of CH3NH2 in water. Estimate the percent ionization of CH3NH2 in a 0.050 M CH3NH2(aq) solution. (Kb for CH3NH2 = 4.4 × 10-4)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:00
How many mmols of tris-hcl are there in 100 ml of a 100 mm tris-hcl buffer solution at ph 8.1? note that the 100 mm refers to the sum of tris and tris-hcl concentrations?
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3


Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
Consider the chemical equation for the ionization of CH3NH2 in water. Estimate the percent ionizatio...
Questions

Mathematics, 30.10.2020 17:40





Mathematics, 30.10.2020 17:40


Mathematics, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40


Computers and Technology, 30.10.2020 17:40


Mathematics, 30.10.2020 17:40



Mathematics, 30.10.2020 17:40

Mathematics, 30.10.2020 17:40


Biology, 30.10.2020 17:40

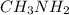 in a 0.050 M
in a 0.050 M 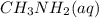 solution is 8.9 %
solution is 8.9 %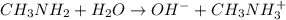
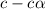
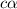
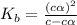
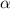 = degree of ionisation = ?
= degree of ionisation = ?