Chemistry, 19.03.2020 17:08 Levantine3667
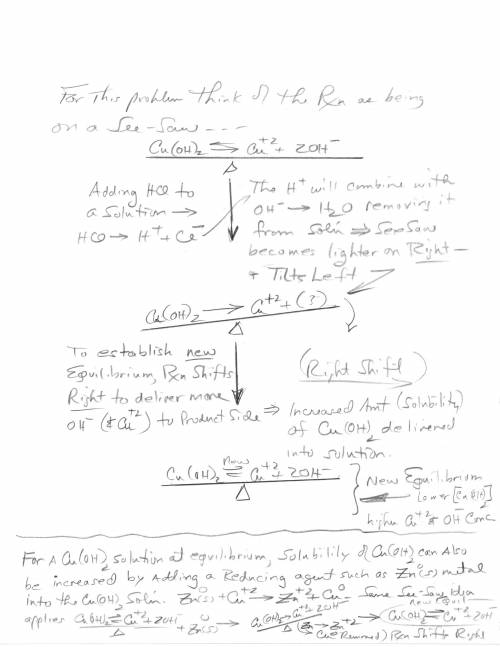
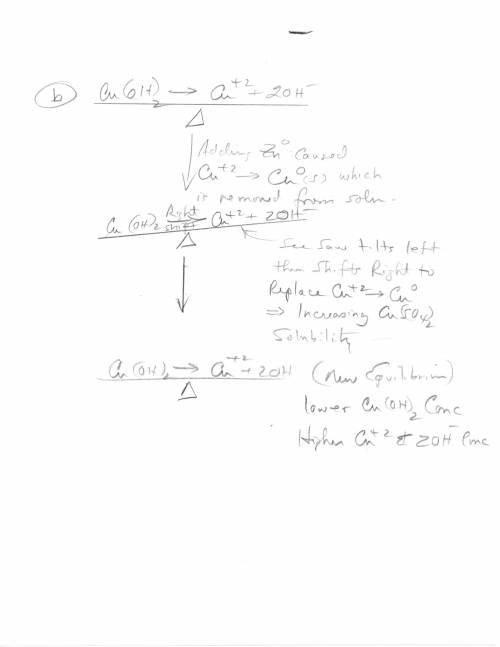
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly soluble. Cu(OH)2(s) Cu2 (aq) 2OH- (aq) a. Explain how the solubility can be increased by adding HCl to the solution. b. Explain how the concentration of copper (II) ion or of hydroxide ion can be reduced in the solution so that more of the solid copper hydroxide can be dissolved.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1

Chemistry, 22.06.2019 14:00
How is the atomic number of a nucleus changed by alpha decay
Answers: 2

You know the right answer?
3. Copper hydroxide dissolves in water according to the following equation. It is only very slightly...
Questions



Biology, 29.01.2020 11:51

Mathematics, 29.01.2020 11:51

History, 29.01.2020 11:51

English, 29.01.2020 11:51







History, 29.01.2020 11:52


Computers and Technology, 29.01.2020 11:52


Mathematics, 29.01.2020 11:52


Mathematics, 29.01.2020 11:52

Mathematics, 29.01.2020 11:52