
Chemistry, 19.03.2020 09:31 priscillarios30
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g) Calculate the equilibrium concentrations of reactant and products when 0.372 moles of HI are introduced into a 1.00 L vessel at 698 K. [HI] = M [H2] = M [I2] = M

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Given the table below, what is the chemical formula for a compound between rb and the nitrate ion no3 -1? nitrate ion no3 -1 phosphate po4 -3 sulfate so4 -2 acetate c2h3o2 -1 ammonium nh4 +1 chromate cro4 -2 carbonate co3 -2 dichromate cr2o7 -2 permanganate mno4 -1 sulfite so3 -2 rbno3 rb2no3 rb(no3)2 rb2(no3)3
Answers: 1

Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1


Chemistry, 23.06.2019 00:40
To prevent the presence of air, noble gases are placed over highly reactive chemicals to act as inert "blanketing" gases. a chemical engineer places a mixture of noble gases consisting of 4.37 g of he, 13.36 g of ne, and 36.65 g of kr in a piston-cylinder assembly at stp. calculate the partial pressure in torr of kr.
Answers: 1
You know the right answer?
The equilibrium constant, Kc, for the following reaction is 1.80×10-2 at 698 K. 2HI(g) H2(g) + I2(g)...
Questions

Mathematics, 12.08.2021 20:10


Mathematics, 12.08.2021 20:10


Biology, 12.08.2021 20:10

Mathematics, 12.08.2021 20:10

Mathematics, 12.08.2021 20:10

History, 12.08.2021 20:10




Mathematics, 12.08.2021 20:10

English, 12.08.2021 20:10

Mathematics, 12.08.2021 20:10

History, 12.08.2021 20:10


Chemistry, 12.08.2021 20:20



![[HI]=(0.372-2x) M =(0.372-2\times 0.03935)M =0.2933 M](/tpl/images/0553/9717/216fb.png)
![[H_2]=x = 0.03935 M](/tpl/images/0553/9717/0d5d5.png)
![[I_2]=x = 0.03935 M](/tpl/images/0553/9717/d2ad6.png)
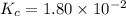
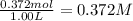
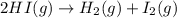
![K_c=\frac{[H_2][I_2]}{[HI]^2}](/tpl/images/0553/9717/ef85e.png)



