Chemistry, 19.03.2020 08:57 cristianTalonzo
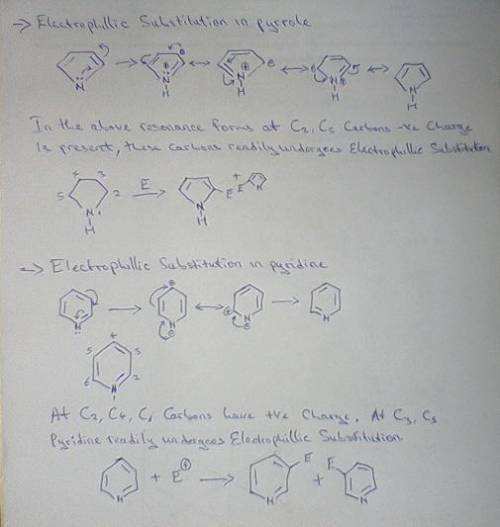
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (EAS). Using a resonance argument, predict the regiochemistry of EAS on both pyrrole and pyridine. As part of your argument, you must draw resonance structures for the arenium ion leading to each possible regiochemistry, and compare the stabilities of the resonance hybrids. In addition, make a reasoned statement about whether you think each one would react faster or slower than benzene and why.

Answers: 2


Another question on Chemistry


Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

You know the right answer?
Both pyrrole and pyridine are aromatic compounds, and undergo electrophilic aromatic substitution (E...
Questions




Mathematics, 10.03.2021 23:10



Mathematics, 10.03.2021 23:10

Mathematics, 10.03.2021 23:10


Mathematics, 10.03.2021 23:10

Physics, 10.03.2021 23:10



Mathematics, 10.03.2021 23:10


Mathematics, 10.03.2021 23:10


Mathematics, 10.03.2021 23:10

Mathematics, 10.03.2021 23:10