
Chemistry, 19.03.2020 08:29 RealSavage4Life
P is the pressure in atmospheres (atm), V is the volume in liters (L), n is the number of moles, R is the gas constant (0.0821 L∙atm/(mol∙K)), and T is the temperature in Kelvins (K). Consider the following conditions: a sample of neon gas was under 3.0 atm of pressure, a volume of 570 mL with a temperature of 75 °C. Assume you are going to use the ideal gas law to solve for the unknown variable. What variable are you solving for? Are all of variables in the correct units? If not, which variable needs to be converted to the correct units?

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 05:00
Type the letter that represents the correct location for each particle type below.
Answers: 1

Chemistry, 22.06.2019 08:30
In the reaction between a crushed antacid tablet and vinegar what gas is emitted
Answers: 2

Chemistry, 22.06.2019 13:50
Amap that uses a range of colors and shading to represent the elevation, depth, or landscape of specific features on earth is a/an map.
Answers: 3
You know the right answer?
P is the pressure in atmospheres (atm), V is the volume in liters (L), n is the number of moles, R i...
Questions

Geography, 15.07.2019 23:40



Mathematics, 15.07.2019 23:40

History, 15.07.2019 23:50

Chemistry, 15.07.2019 23:50



Spanish, 15.07.2019 23:50


Mathematics, 15.07.2019 23:50

History, 15.07.2019 23:50





Physics, 15.07.2019 23:50


Physics, 15.07.2019 23:50

Physics, 15.07.2019 23:50

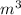

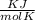
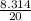 = 0.41
= 0.41 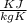
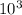 × 0.00057 = m × 0.41 × 348
× 0.00057 = m × 0.41 × 348 

