
A piece of iron (mass = 25.0 g) at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of water at 298 K. Assuming that no heat is lost to the cup or the surroundings, what will the final temperature of the water be? The specific heat capacity of iron = 0.449 J/g°C and water = 4.18 J/g°C.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which term best describes the form sound takes as it travels away from a drum (a- gas)(b-music) ( c-waves) (d-particles
Answers: 3

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 18:00
The fact that the total amount of energy in a system remains constant is a(n)
Answers: 1

Chemistry, 22.06.2019 19:00
Avolleyball player hit a ball with a mass of 0.25 kg. the average acceleration of the ball is 15.5 m/s². how much force did the volleyball player apply to the ball? 62.0 n 3.87 n 62.0 m/s² 3.87 m/s²
Answers: 2
You know the right answer?
A piece of iron (mass = 25.0 g) at 398 K is placed in a styrofoam coffee cup containing 25.0 mL of w...
Questions

SAT, 26.06.2019 04:30

Physics, 26.06.2019 04:30

Biology, 26.06.2019 04:30


Geography, 26.06.2019 04:30







Mathematics, 26.06.2019 04:30

Mathematics, 26.06.2019 04:30


Mathematics, 26.06.2019 04:30



Mathematics, 26.06.2019 04:30

History, 26.06.2019 04:30

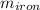 = 25 gm
= 25 gm = 398 K
= 398 K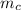 = 25 ml = 25 gm
= 25 ml = 25 gm = 298 K
= 298 K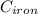 = 0.449
= 0.449 
 = 4.18
= 4.18  =
=  ×
× 
 = 25 × 4.18 ×
= 25 × 4.18 × 
 = 9.3 (
= 9.3 (  = 9.3
= 9.3 

