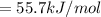Chemistry, 19.03.2020 05:55 bryanmcmillianjr
Consider the Gibbs energies at 25 ∘ C. Substance Δ G ∘ f ( kJ ⋅ mol − 1 ) Ag + ( aq ) 77.1 Cl − ( aq ) − 131.2 AgCl ( s ) − 109.8 Br − ( aq ) − 104.0 AgBr ( s ) − 96.9 (a) Calculate Δ G ∘ rxn for the dissolution of AgCl ( s ) .

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 10:00
Ahydrogen atom has 1 electron. how many bonds can hydrogen form? a) 1 b) 2 c) 3 d) 4 e) 5
Answers: 3


Chemistry, 22.06.2019 17:00
Complete each row of the table below by filling in the missing prefix or missing exponent.
Answers: 1
You know the right answer?
Consider the Gibbs energies at 25 ∘ C. Substance Δ G ∘ f ( kJ ⋅ mol − 1 ) Ag + ( aq ) 77.1 Cl − ( aq...
Questions


Mathematics, 21.12.2020 07:30

Advanced Placement (AP), 21.12.2020 07:30


Arts, 21.12.2020 07:30


Mathematics, 21.12.2020 07:30

English, 21.12.2020 07:30

History, 21.12.2020 07:30

Biology, 21.12.2020 07:30

English, 21.12.2020 07:30

Mathematics, 21.12.2020 07:30


Business, 21.12.2020 07:30

Advanced Placement (AP), 21.12.2020 07:30

English, 21.12.2020 07:30

English, 21.12.2020 07:30

History, 21.12.2020 07:30


Mathematics, 21.12.2020 07:30

![\Delta G_{rxn}^o=\sum[\Delta G^o_{f}]_{products}-\sum[\Delta G^o_{f}]_{reactants}](/tpl/images/0553/6074/2b279.png)
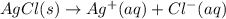
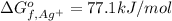
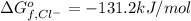
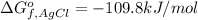
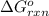 :
:![\Delta G_{rxn}^o=[77.1 kJ/mol+(-131.2 kJ/mol)]-[-109.8 kJ/mol]](/tpl/images/0553/6074/68588.png)