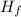Chemistry, 19.03.2020 05:38 Marshmallow9174
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75.2 J/mol⋅KJ/mol⋅K and that the heat capacity of H2O(s)H2O(s) is 37.7 J/mol⋅KJ/mol⋅K, calculate the heat of fusion of water at -15 ∘C∘C.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
What are the three major branches of natural science? • earth and space science, life science, physical science •earth and space science, physical science, chemistry •physical science, life science, chemistry •life science, chemistry, physics
Answers: 1

Chemistry, 22.06.2019 04:30
Which statement best describes the relationship between period and frequency of light waves? a) in wave b the period increases and the frequency decreases from wave a. b) in wave a the period increases and the frequency decreases from wave b. c) in wave b the period is shorter and the frequency is greater than in wave a. d) in wave a the period is shorter and the frequency is greater than in wave b.
Answers: 1

Chemistry, 22.06.2019 06:00
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 15:00
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
You know the right answer?
Given that the heat of fusion of water is +6.02 kJ/mol that the heat capacity of H2O(l)H2O(l) is 75....
Questions





History, 18.01.2020 17:31

Mathematics, 18.01.2020 17:31



Mathematics, 18.01.2020 17:31

Mathematics, 18.01.2020 17:31

Mathematics, 18.01.2020 17:31


History, 18.01.2020 17:31







English, 18.01.2020 17:31

 ·(T₂ - T₁)
·(T₂ - T₁)