
Chemistry, 19.03.2020 03:48 kaywendel2008
For the reaction hconh2(g)⇌nh3(g)+co(g), k= 4.84 at 400 k what can be said about this reaction at this temperature? view available hint(s) for the reaction , at 400 what can be said about this reaction at this temperature? the equilibrium lies far to the right. the reaction will proceed very slowly. the reaction contains significant amounts of products and reactants at equilibrium. the equilibrium lies far to the left.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:30
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 22.06.2019 01:00
Which type of orbits are found in the principal energy level n = 2 a - s b - s, f c - s, d d - s, p e - s, p, d
Answers: 1

Chemistry, 22.06.2019 05:30
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
For the reaction hconh2(g)⇌nh3(g)+co(g), k= 4.84 at 400 k what can be said about this reaction at th...
Questions

Mathematics, 22.06.2021 05:00

Biology, 22.06.2021 05:00


Mathematics, 22.06.2021 05:00



Mathematics, 22.06.2021 05:00

Mathematics, 22.06.2021 05:00

English, 22.06.2021 05:00



Mathematics, 22.06.2021 05:00

Social Studies, 22.06.2021 05:00

Chemistry, 22.06.2021 05:00

Mathematics, 22.06.2021 05:00

Mathematics, 22.06.2021 05:10


Physics, 22.06.2021 05:10

History, 22.06.2021 05:10

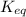
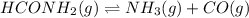
![K_{eq}=\frac{[NH_3]\times [CO]}{[HCONH_2]}](/tpl/images/0553/4863/2c193.png)
![4.84=\frac{[NH_3]\times [CO]}{[HCONH_2]}](/tpl/images/0553/4863/48469.png)



