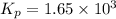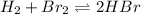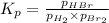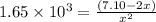Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 11:00
The number to the right of an element's symbol (ex. c-12) identifies the of an isotope.
Answers: 1

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 13:30
If the concentration of phosphate in the cytosol is 2.0 mm and the concentration of phosphate in the surrounding fluid is 0.1 mm, how could the cell increase the concentration of phosphate in the cytosol? a) passive transportb) diffusionc) active transportd) osmosise) facilitated diffusion
Answers: 3
You know the right answer?
The equilibrium constant for the following reaction: H2(g) + Br2(g) ↔ 2HBr (g) is 1.65 x 103 at a ce...
Questions


English, 27.09.2021 18:10




Arts, 27.09.2021 18:10

Mathematics, 27.09.2021 18:10





Mathematics, 27.09.2021 18:10

Mathematics, 27.09.2021 18:10

English, 27.09.2021 18:10






Mathematics, 27.09.2021 18:10