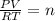Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 20:30
We are hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 3

Chemistry, 22.06.2019 21:30
The solid xy decomposes into gaseous x and y: xy(s) m x(g) + y(g) kp = 4.1 (at 0 °c) if the reaction is carried out in a 22.4 l container, which initial amounts of x and y will result in the formation of solid xy?
Answers: 1
You know the right answer?
I need help with this question...
The equation for methane is ( CH4(g) + 2O2(g) --> 2...
The equation for methane is ( CH4(g) + 2O2(g) --> 2...
Questions



Mathematics, 25.01.2021 23:10


Biology, 25.01.2021 23:10

Mathematics, 25.01.2021 23:10

Mathematics, 25.01.2021 23:10

Mathematics, 25.01.2021 23:10

Mathematics, 25.01.2021 23:10


Mathematics, 25.01.2021 23:10

Advanced Placement (AP), 25.01.2021 23:10


Mathematics, 25.01.2021 23:10

English, 25.01.2021 23:10



Mathematics, 25.01.2021 23:10

Computers and Technology, 25.01.2021 23:10