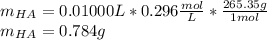Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Which of the following statements is true? a. elements in the last period are radioactive. b. atomic weight is the same as atomic mass. c. elements in the same group have the same number of electron shells. d. atomic number equals the number of neutrons in the nucleus of an atom.
Answers: 1

Chemistry, 22.06.2019 06:30
Type the correct answer in the box. spell all words correctly.what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1

Chemistry, 22.06.2019 08:00
Match the mixture with the substance// i really need on this guys (it’s a pic btw)
Answers: 1
You know the right answer?
Thiamine hydrochloride (C12H18ON4SCl2) is a water-soluble form of thiamine (vitamin B1; Ka = 3.37×10...
Questions


English, 21.02.2021 02:50

Advanced Placement (AP), 21.02.2021 02:50



Mathematics, 21.02.2021 02:50

Mathematics, 21.02.2021 02:50

Mathematics, 21.02.2021 02:50

English, 21.02.2021 02:50

Mathematics, 21.02.2021 02:50



Mathematics, 21.02.2021 02:50

Mathematics, 21.02.2021 02:50






Mathematics, 21.02.2021 02:50

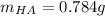
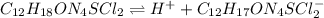
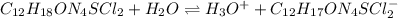
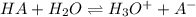
![pH=pKa+log(\frac{[A^-]}{[HA]} )](/tpl/images/0553/3571/4a01a.png)
![log(\frac{[A^-]}{[HA]} )=3.50-[-log(3.37x 10^{-7})]=3.50-6.47=-2.97}\\\\\frac{[A^-]}{[HA]} =10^{-2.97}=1.07x10^{-3}](/tpl/images/0553/3571/b4cdb.png)
![[A^-]=1.07x10^{-3}}[HA]](/tpl/images/0553/3571/1acb8.png)
![[H]^+=[A^-]=10^{-pH}=10^{-3.50}=3.16x10^{-4}M](/tpl/images/0553/3571/d3a0e.png)
![[HA]=\frac{3.16x10^{-4}M}{1.07x10^{-3}} =0.296M](/tpl/images/0553/3571/7981c.png)