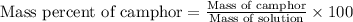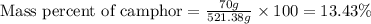Chemistry, 19.03.2020 01:36 briseisr20
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the camphor tree. Assume you dissolve 70.0 g of camphor (C10H16O) in 575 mL of ethanol, C2H5OH. Calculate the molarity, molality, mole fraction, and weight percentage of camphor in this solution. (The density of ethanol is 0.785 g/mL.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 09:00
Achemist 16 drop copper metal from copper chloride solution. the chemist place is 0.50 g of aluminum foil in a solution containing 0.75 g of copper (ii) chloride. a single replacement reaction takes place. which statement explains the maximum amount of copper that the chemist can extract using this reaction?
Answers: 1

Chemistry, 22.06.2019 22:10
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:30
Which statement best describes the liquid state of matter? a. it has definite shape but indefinite volume. b. it has definite shape and definite volume. c. it has indefinite shape and indefinite volume. d. it has indefinite shape but definite volume.
Answers: 1
You know the right answer?
Camphor, a white solid with a pleasant odor, is extracted from the roots, branches, and trunk of the...
Questions



Mathematics, 07.02.2021 01:40

Chemistry, 07.02.2021 01:40

Mathematics, 07.02.2021 01:40

Mathematics, 07.02.2021 01:40

History, 07.02.2021 01:40


Mathematics, 07.02.2021 01:40

Mathematics, 07.02.2021 01:40

Chemistry, 07.02.2021 01:40

Biology, 07.02.2021 01:40


Arts, 07.02.2021 01:40


Mathematics, 07.02.2021 01:40

Mathematics, 07.02.2021 01:40



Arts, 07.02.2021 01:40

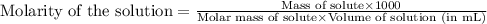
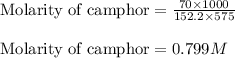
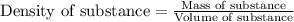
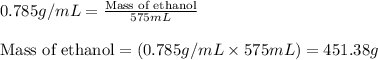
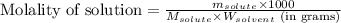
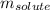 = Given mass of solute (camphor) = 70 g
= Given mass of solute (camphor) = 70 g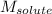 = Molar mass of solute (camphor) = 152.2 g/mol
= Molar mass of solute (camphor) = 152.2 g/mol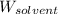 = Mass of solvent (ethanol) = 451.38 g
= Mass of solvent (ethanol) = 451.38 g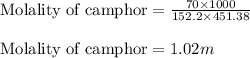
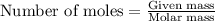 .....(1)
.....(1)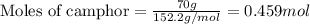
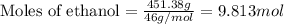
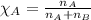
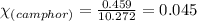 \
\