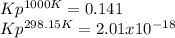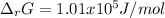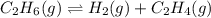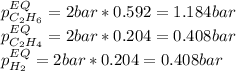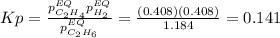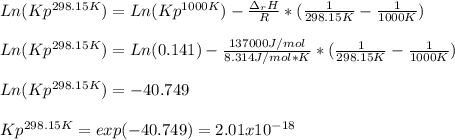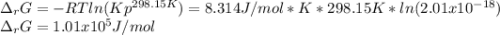Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introd...

Consider the equilibrium
At 1000 K and a const. total pressure of 2 bar, C2H6 is introduced into a reaction vessel. the total pressure is held const. at 2 bar and at equilibrium the composition of the mixture in mole percent is H2(g): 20.4%, C2H4 (g): 20.4%, and C2H6 (g): 59.2%.
Calculate Kp at 1000 K
if ΔH of reaction = 137 kJ/mol, calculate the value of Kp at 298.15K
Calculate ΔG of reaction for this reaction at 298.15 K

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Fission of uranium-235 products energy and a. isotopes of smaller elements b. isotopes of larger elements c. lighter isotopes of uranium d. heavier isotopes of uranium
Answers: 3

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2


Chemistry, 23.06.2019 08:00
Which of the following notations would be the appropriate and final way to display the formula for magnesium chloride a. mgcl2 b. mg+2cl–1 c. mgcl2 d. mgcl
Answers: 2
You know the right answer?
Questions


Social Studies, 16.10.2019 20:30

History, 16.10.2019 20:30





Mathematics, 16.10.2019 20:30

History, 16.10.2019 20:30

Geography, 16.10.2019 20:30

Biology, 16.10.2019 20:30

Mathematics, 16.10.2019 20:30



Mathematics, 16.10.2019 20:30


Mathematics, 16.10.2019 20:30

Mathematics, 16.10.2019 20:30

Biology, 16.10.2019 20:30

History, 16.10.2019 20:30