Chemistry, 19.03.2020 01:16 blackwhiteroses383
Consider the reaction. 2 HBr(g) ¡ H2(g) + Br2(g) a. Express the rate of the reaction in terms of the change in concentration of each of the reactants and products. b. In the first 25.0 s of this reaction, the concentration of HBr drops from 0.600 M to 0.512 M. Calculate the average rate of the reaction during this time interval. c. If the volume of the reaction vessel in part b is 1.50 L, what amount of Br2 (in moles) forms during the first 15.0 s of the reaction?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:00
3. if the dartboard below is used to model an atom, which subatomic particles would be located at z?
Answers: 2

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 04:30
Why are people not able to scuba dive in the deep part of the ocean
Answers: 2
You know the right answer?
Consider the reaction. 2 HBr(g) ¡ H2(g) + Br2(g) a. Express the rate of the reaction in terms of the...
Questions




Mathematics, 19.02.2021 21:20


Mathematics, 19.02.2021 21:20

History, 19.02.2021 21:20


Mathematics, 19.02.2021 21:20

Business, 19.02.2021 21:20



Mathematics, 19.02.2021 21:20



Biology, 19.02.2021 21:20

English, 19.02.2021 21:20

English, 19.02.2021 21:20

Social Studies, 19.02.2021 21:20

Mathematics, 19.02.2021 21:20

![Rate=-\frac{1}{2}\frac{d[HBr]}{dt}=+\frac{d[H_2]}{dt}=+\frac{d[Br_2]}{dt}](/tpl/images/0553/1566/27c4e.png)
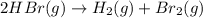
![\text{Rate of disappearance of }HBr=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0553/1566/d63dd.png)
![\text{Rate of disappearance of }H_2=+\frac{d[H_2]}{dt}](/tpl/images/0553/1566/eb73c.png)
![\text{Rate of formation of }Br_2=+\frac{d[Br_2]}{dt}](/tpl/images/0553/1566/30b3c.png)
![\text{Average rate}=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0553/1566/79555.png)
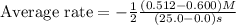
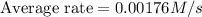
![\frac{d[Br_2]}{dt}=0.00176M/s](/tpl/images/0553/1566/78ef0.png)
![\frac{d[Br_2]}{15.0s}=0.00176M/s](/tpl/images/0553/1566/22daf.png)
![[Br_2]=0.00176M/s\times 15.0s](/tpl/images/0553/1566/5d9c4.png)
![[Br_2]=0.0264M](/tpl/images/0553/1566/42226.png)