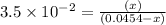Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10−2 If you start with 25.0 mLmL of a 0.909 MM solution of NaIO4NaIO4, and then dilute it with water to 500.0 mLmL, what is the concentration of H4IO−6H4IO6− at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 07:30
In the particles are arranged in a regular, repeating pattern. a)a crystalline liquid b)a crystalline solid c)all gases d)all solids
Answers: 2

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1


Chemistry, 22.06.2019 18:00
Answer asap need to be answered by wednesday morning explain how a buffer works, using an ethanoic acid / sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 3
You know the right answer?
Consider the reaction IO−4(aq)+2H2O(l)⇌H4IO−6(aq);Kc=3.5× 10−2IO4−(aq)+2H2O(l)⇌H4IO6−(aq);Kc= 3.5×10...
Questions



Mathematics, 18.03.2020 06:10

Mathematics, 18.03.2020 06:10

Spanish, 18.03.2020 06:10









Mathematics, 18.03.2020 06:11

English, 18.03.2020 06:11


Mathematics, 18.03.2020 06:11



Computers and Technology, 18.03.2020 06:11

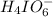 at equilibrium is, 0.00154 M
at equilibrium is, 0.00154 M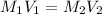
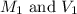 are the initial molarity and volume of
are the initial molarity and volume of 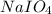 .
.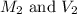 are the final molarity and volume of diluted
are the final molarity and volume of diluted 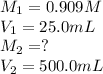
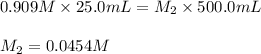
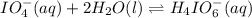
![K_c=\frac{[H_4IO_6^-]}{[IO_4^-]}](/tpl/images/0553/1589/ffca5.png)