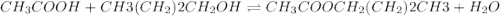Which one of the following choices describes most accurately the actual, internal reaction temperature (in other words, the temperature of the reaction mixture inside the reaction vial) for the Fischer esterification experiment of 1-butanol with acetic acid to form n-butyl acetate? Select one, and explain your answer.
a) Sand bath temperature (160-180 °C)
b) Boiling point of 1-butanol (116-118 °C)
c) Boiling point of the reaction mixture (reflux temperature)
d) Boiling point of acetic acid (117 °C)
e) Boiling point of n-butyl acetate (124-126 °C)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
If 10.g of agno3 is available, what volume of 0.25 m agno3 can be prepared
Answers: 1

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 13:00
What is the mass of 2.00 l of an intravenous glucose solution with a density of 1.15 g/ml?
Answers: 2

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
You know the right answer?
Which one of the following choices describes most accurately the actual, internal reaction temperatu...
Questions






Mathematics, 11.12.2019 22:31


Mathematics, 11.12.2019 22:31






Mathematics, 11.12.2019 22:31