
Chemistry, 19.03.2020 00:41 allieballey0727
A generic metal thiocyanate, M(SCN)2, has a Ksp value of 2.00×10−5. Calculate the molar solubility of the metal thiocyanate in 0.421 M KSCN. Express your answer numerically in units of mM to 4 decimal places.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
1) these are barrel shaped microtubules in most animal cells, that organize the spindles during cell division
Answers: 1

Chemistry, 22.06.2019 15:30
How does a large body of water, such as the ocean, influence climate?
Answers: 1

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 3

Chemistry, 23.06.2019 05:00
Question 5 match each term to its description. match term definition excess reactant a) reactant that can produce a lesser amount of the product limiting reactant b) reactant that can produce more of the product theoretical yield c) amount of product predicted to be produced by the given reactants
Answers: 2
You know the right answer?
A generic metal thiocyanate, M(SCN)2, has a Ksp value of 2.00×10−5. Calculate the molar solubility o...
Questions

Mathematics, 19.11.2020 03:10



Mathematics, 19.11.2020 03:10

English, 19.11.2020 03:10

History, 19.11.2020 03:10

Mathematics, 19.11.2020 03:10

Mathematics, 19.11.2020 03:10


Mathematics, 19.11.2020 03:10

Spanish, 19.11.2020 03:10

Mathematics, 19.11.2020 03:10

Engineering, 19.11.2020 03:10

Mathematics, 19.11.2020 03:10



English, 19.11.2020 03:10

Advanced Placement (AP), 19.11.2020 03:10

Computers and Technology, 19.11.2020 03:10

Mathematics, 19.11.2020 03:10

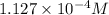 .
.![[SCN^-]= 0.421 M](/tpl/images/0553/0411/3a7d7.png)
![[M^{2+}]= ?](/tpl/images/0553/0411/05fb9.png)
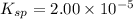
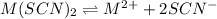
![K_{sp}=[M^{2+}]\times [SCN^-]^2](/tpl/images/0553/0411/0d18b.png)
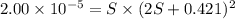
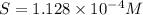
![[M^{2+}]=\frac{2.00\times 10^{-5}}{(0.421 M)^2}=1.127\times 10^{-4} M](/tpl/images/0553/0411/8e36e.png)



