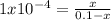Chemistry, 18.03.2020 19:39 JellalFernandes
A reaction A(g)⇌B(g)has an equilibrium constant of 1.0×10−4 For which of the initial reaction mixtures is the small approximation most likely to apply? Explain why.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:10
Here’s one way to follow the scientific method. place the missing steps in the correct position in the process
Answers: 1

Chemistry, 22.06.2019 02:00
If you add 10ml of hot water to 10ml of cold water and the change in tempature 8°c calculate how much energy is gained by the cold water
Answers: 1


Chemistry, 22.06.2019 04:30
What are the primary responsibilities of a chemical engineer involved in "r& d"? develop large scale manufacturing operations discover new products and processes training of new chemists determine products needed by consumers
Answers: 2
You know the right answer?
A reaction A(g)⇌B(g)has an equilibrium constant of 1.0×10−4 For which of the initial reaction mixtur...
Questions







Social Studies, 28.01.2020 00:31













 in this case would be 0.00001M which is the smallest one from the given options, considering:
in this case would be 0.00001M which is the smallest one from the given options, considering: