Chemistry, 18.03.2020 18:57 dyllanmasters99
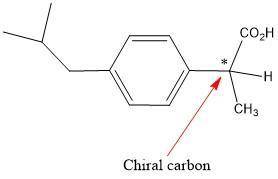
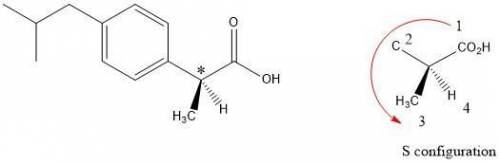
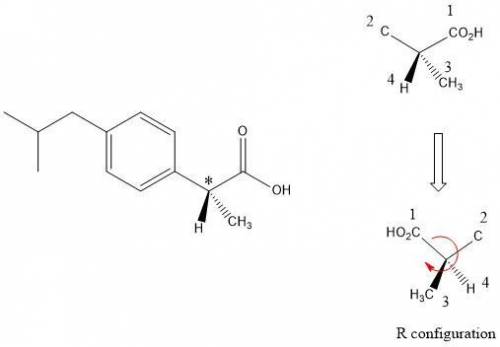
Buprofen, a well-known non-steroidal anti-inflammatory drug, has chirality. Only the S enantiomer has anti-inflammatory activity (although the R enantiomer is converted slowly by the body into the S enantiomer). Add wedge-and-dash bonds to complete the perspective structures of the two stereoisomers of ibuprofen.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Urea, co(nh2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. what is the maximum mass of urea that can be manufactured from the co2 produced by combustion of 1.00 x 104 grams of co2?
Answers: 1

Chemistry, 22.06.2019 00:30
Elements that do not have full outer electron shells will donate, share, or take electrons from other atoms. choose the items that have the correct binary ionic formula.
Answers: 2

Chemistry, 22.06.2019 13:00
How many moles of sulfur dioxide are produced when 4.38 moles of oxygen completely react with iron (iv) sulfide
Answers: 2

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2
You know the right answer?
Buprofen, a well-known non-steroidal anti-inflammatory drug, has chirality. Only the S enantiomer ha...
Questions

History, 11.06.2020 07:57

History, 11.06.2020 07:57



Mathematics, 11.06.2020 07:57



Mathematics, 11.06.2020 07:57






Mathematics, 11.06.2020 07:57

Biology, 11.06.2020 07:57


Business, 11.06.2020 07:57