
Chemistry, 18.03.2020 18:32 laceysmith2i023
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or Lewis acid/base reaction. Group of answer choices Only Bronsted-Lowry Only Lewis Both Bronsted-Lowry and Lewis Neither

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:30
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 22:30
3.09 lab: reaction of metals 1 which combinations of substances resulted in a chemical change? for each metal that participated in a chemical change, write the type of metal it is, based on your examination of the periodic table. were there any metallic compounds that did not react with either the acid or the base? write the type of metal, based on your examination of the periodic table. make a general statement about the reactivity of the metals in this experiment.
Answers: 1


Chemistry, 23.06.2019 11:00
The decimals you found in part b are called repeating decimals. in the gizmo, repeating decimals are rounded to two places. how does the gizmo show you that a decimal has been rounded?
Answers: 3
You know the right answer?
Al(OH)3 + OH- → Al(OH)4- Identify whether this reaction can be classified as a Bronsted-Lowry and/or...
Questions


Mathematics, 17.03.2020 00:27

Mathematics, 17.03.2020 00:27


Mathematics, 17.03.2020 00:27


Computers and Technology, 17.03.2020 00:27









Biology, 17.03.2020 00:27




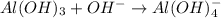
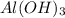 is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.
is a Lewis acid because it is an electron deficient species that means, it accepts electron pairs.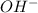 is a Lewis base because it is an electron rich species that means, it donates electron pairs.
is a Lewis base because it is an electron rich species that means, it donates electron pairs.

