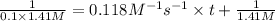At a certain temperature this reaction follows second-order kinetics with a rate constant of 0.118 M^-1* s^-1:
ClCH2CH2Cl (g) > CH2CHCI(g) + HCl (g)
a) Suppose a vessel contains ClCH2CH2Cl at a concentration of 1.41 M. Calculate how long it takes for the concentration of CICH2CH2Cl to decrease to 10.0% of its initial value. You may assume no other reaction is important. Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1


Chemistry, 22.06.2019 04:30
Both josef loschmidt and amedeo avogadro contributed to our understanding of basic molecular numbers, sizes, and reaction ratios. neither scientist discovered “avogadro’s number” in the form we use it today (6.02 x 10 23). still, there’s a controversy over the name. research the contributions from these two scientists and read about how avogadro’s number got its name. briefly state what you think this number should be called, providing key details of each scientist’s contributions to this concept and a solid rationale for your case in naming the number.
Answers: 2

Chemistry, 22.06.2019 05:30
Arecipe calls for 1.2 cups of oil. how many liters of oil is this?
Answers: 2
You know the right answer?
At a certain temperature this reaction follows second-order kinetics with a rate constant of 0.118 M...
Questions

Biology, 13.03.2021 02:30


Computers and Technology, 13.03.2021 02:30

Biology, 13.03.2021 02:30


Mathematics, 13.03.2021 02:30



English, 13.03.2021 02:30

Arts, 13.03.2021 02:30


Health, 13.03.2021 02:30


English, 13.03.2021 02:30

English, 13.03.2021 02:30





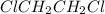 to decrease to 10.0% of its initial value.
to decrease to 10.0% of its initial value.![[A_o]=1.41 M](/tpl/images/0551/6145/c4ebe.png)
![[A]=10\%of [A_o]=0.1[A_o]](/tpl/images/0551/6145/99a59.png)
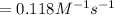
![\frac{1}{[A]}=kt+\frac{1}{[A_o]}](/tpl/images/0551/6145/a4900.png)