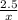2. How many grams of Potassium Chloride, KCl, are produced if 25.0 g of Potassium Chlorate, KClO3, decompose?
2KClO3 → 2KCl + 3O2
3. What is the moles of H2 gas produced when 100.0 grams of Na is added to the reaction?
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
4. How many moles of Hydrogen, H2, are needed to react with 2.5 moles of Nitrogen, N2?
N2 + 3H2 → 2NH3

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 22.06.2019 21:30
How can the periodic table be used to predict the behavior of elements?
Answers: 1
You know the right answer?
2. How many grams of Potassium Chloride, KCl, are produced if 25.0 g of Potassium Chlorate, KClO3, d...
Questions





Mathematics, 07.10.2020 23:01













English, 07.10.2020 23:01


Biology, 07.10.2020 23:01

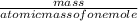
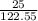
 =
= 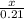
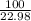
 =
= 
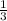 =
=