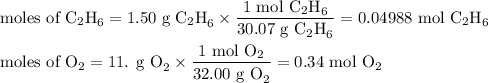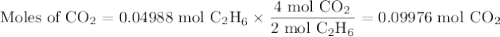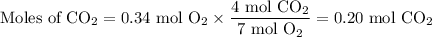Chemistry, 18.03.2020 00:42 bravomichelle75
Gaseous ethane (CH, CH,) will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO) and gaseous water (H2O). Suppose 1.50 g of
ethane is mixed with 11. g of oxygen. Calculate the minimum mass of ethane that could be left over by the chemical reaction. Round your answer to 2
significant digits.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 14:10
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 19:30
What is the common name for the compound shown here? enter the common name of the compound shown?
Answers: 2

Chemistry, 22.06.2019 21:30
What is the correct name for the compound cocl3? a) cobalt(i) chloride b) cobalt(i) chlorate c) cobalt(ii) chlorate d) cobalt(iii) chloride
Answers: 1
You know the right answer?
Gaseous ethane (CH, CH,) will react with gaseous oxygen (0,) to produce gaseous carbon dioxide (CO)...
Questions




History, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01



Business, 04.09.2020 14:01

Mathematics, 04.09.2020 14:01

History, 04.09.2020 14:01




Mathematics, 04.09.2020 14:01

Physics, 04.09.2020 14:01

Social Studies, 04.09.2020 14:01


Mathematics, 04.09.2020 14:01


Mathematics, 04.09.2020 14:01