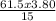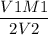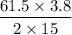Chemistry, 18.03.2020 00:28 probablyacommunist
If you used 61.5mL of 3.80 M KOH to titrate 15.0 ML of H2SO4, what was the concentration of the H2SO4?.

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
How does the presence of oxygen affect the chemical pathways used to extract energy from glucose?
Answers: 3

Chemistry, 22.06.2019 16:10
Predict the reactants of this chemical reaction. that is, fill in the left side of the chemical equation. be sure the equation you submit is balanced. (you can edit both sides of the equation to balance it, if you need to.) note: you are writing the molecular, and not the net ionic equation. > cacl2(aq) + h20(l)
Answers: 2

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 2
You know the right answer?
If you used 61.5mL of 3.80 M KOH to titrate 15.0 ML of H2SO4, what was the concentration of the H2SO...
Questions





English, 12.08.2020 08:01

Health, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01

Biology, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01


Social Studies, 12.08.2020 08:01

English, 12.08.2020 08:01


Mathematics, 12.08.2020 08:01

Mathematics, 12.08.2020 08:01


Medicine, 12.08.2020 08:01



English, 12.08.2020 08:01