
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? The reaction described in Part A required 3.36 L of calcium chloride. What is the concentration of this calcium chloride solution?
Please explain, I don't know where to begin.
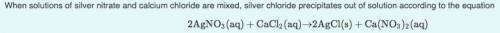

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
This question is about electrolysis. metal spoons can be coated with silver. this is called electroplating. suggest one reason why spoons are electroplated?
Answers: 1

Chemistry, 22.06.2019 10:00
Nonpoint source pollution is difficult to control because it
Answers: 2


Chemistry, 22.06.2019 23:00
What is the number of neutrons in an atom with atomic mass of 35
Answers: 2
You know the right answer?
What mass of silver chloride can be produced from 1.30 L of a 0.245 M solution of silver nitrate? Th...
Questions

Mathematics, 24.07.2019 18:30


Mathematics, 24.07.2019 18:30


Mathematics, 24.07.2019 18:30

Physics, 24.07.2019 18:30

Geography, 24.07.2019 18:30


English, 24.07.2019 18:30


Mathematics, 24.07.2019 18:30

Mathematics, 24.07.2019 18:30

Mathematics, 24.07.2019 18:30

English, 24.07.2019 18:30

Mathematics, 24.07.2019 18:30



History, 24.07.2019 18:30

Mathematics, 24.07.2019 18:30



