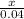Chemistry, 17.03.2020 21:42 dzepeda061
2 AgNO3(aq) + CaCl2(aq) > 2 AgCl(s) + Ca(NO3)2(aq)
How many grams of AgCi are formed when 0.200 L of 0.200 M AGNO3 reacts with an excess of CaCl2?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 13:30
What would happen if no were added to n(g)+o2=2no(g) at equilibrium?
Answers: 1
You know the right answer?
2 AgNO3(aq) + CaCl2(aq) > 2 AgCl(s) + Ca(NO3)2(aq)
How many grams of AgCi are formed...
How many grams of AgCi are formed...
Questions


History, 16.01.2021 21:30




SAT, 16.01.2021 21:40

Mathematics, 16.01.2021 21:40


English, 16.01.2021 21:40

Mathematics, 16.01.2021 21:40

Biology, 16.01.2021 21:40



Geography, 16.01.2021 21:40

English, 16.01.2021 21:40

Mathematics, 16.01.2021 21:40


Biology, 16.01.2021 21:40


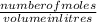
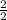 =
=