
Chemistry, 17.03.2020 20:48 sgslayerkingminecraf
A mixture of CO2 and Kr weighs 31.7 g and exerts a pressure of 0.665 atm in its container. Since Kr is expensive, you wish to recover it from the mixture. After the CO2 is completely removed by absorption with NaOH(s), the pressure in the container is 0.309 atm.
(a) How many grams of CO2 were originally present?
(b) How many grams of Kr can you recover?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:40
If 10.0 ml of the solution on the right are withdrawn from the 100 ml beaker and diluted again in a similar manner, what is the new concentration? m nacl
Answers: 2

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3


Chemistry, 22.06.2019 10:00
Suppose the universe were completely empty except for one object-a solid sphere moving through space of 100 km/s. what sort of path would the object be moving in? explain your answer
Answers: 1
You know the right answer?
A mixture of CO2 and Kr weighs 31.7 g and exerts a pressure of 0.665 atm in its container. Since Kr...
Questions

English, 24.02.2021 23:00



Business, 24.02.2021 23:00


Mathematics, 24.02.2021 23:00

Mathematics, 24.02.2021 23:00

Mathematics, 24.02.2021 23:00

Mathematics, 24.02.2021 23:00

Mathematics, 24.02.2021 23:00

English, 24.02.2021 23:00


Mathematics, 24.02.2021 23:00


Biology, 24.02.2021 23:00

English, 24.02.2021 23:00


Mathematics, 24.02.2021 23:00

History, 24.02.2021 23:00

Computers and Technology, 24.02.2021 23:00

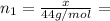
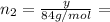
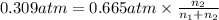
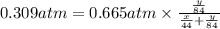
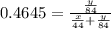 ..[2]
..[2]

