
Chemistry, 17.03.2020 19:53 Asantetaedog8934
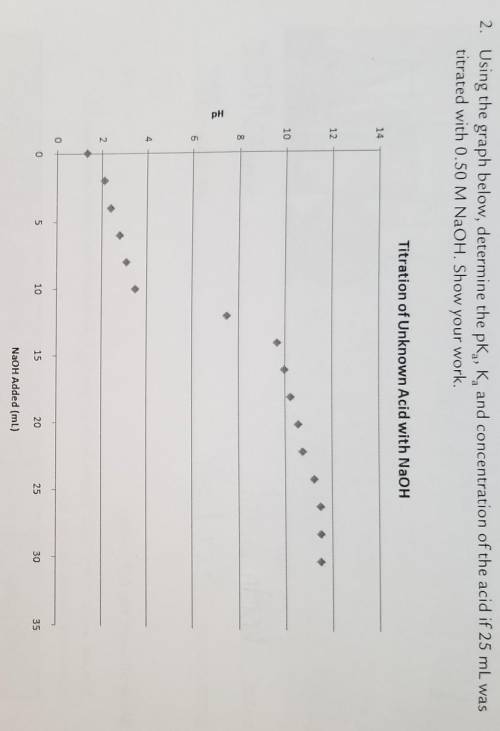
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated woth 0.50 M NaOH. Show your work.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 17:30
The following reaction shows the products when sulfuric acid and aluminum hydroxide react. al(oh)3 + h2so4 → al2(so4)3 + h2o the table shows the calculated amounts of reactants and products when the reaction was conducted in a laboratory. initial mass and yield sulfuric acid aluminum hydroxide initial amount of reactant 40 g 15 g theoretical yield of water from reactant 14.69 g 10.38 g what is the approximate amount of the leftover reactant? 11.73 g of sulfuric acid 10.33 g of sulfuric acid 11.12 g of aluminum hydroxide 13.67 g of aluminum hydroxide
Answers: 1

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 22.06.2019 03:00
What happened in 2012 and how does it illustrate the importance of understanding the sun and how it works?
Answers: 3

Chemistry, 22.06.2019 07:30
Using data from seismic waves, geologists have learned that earth’s interior is made up of several
Answers: 3
You know the right answer?
Using the graph below, determine the pKa, Ka, and concentration of the acid if 25 mL was titrated wo...
Questions

Mathematics, 13.09.2021 17:50

Mathematics, 13.09.2021 17:50






Mathematics, 13.09.2021 17:50



Mathematics, 13.09.2021 18:00

Health, 13.09.2021 18:00


Social Studies, 13.09.2021 18:00

Engineering, 13.09.2021 18:00


Mathematics, 13.09.2021 18:00

Health, 13.09.2021 18:00

Physics, 13.09.2021 18:00



