
Chemistry, 17.03.2020 17:40 tinktkinkdavis7340
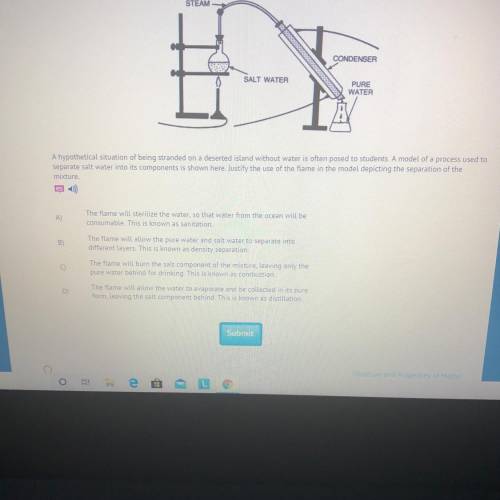
A hypothetical situation of being stranded on a deserted island without water is often posed to students. A model of a process used to
separate salt water into its components is shown here. Justify the use of the flame in the model depicting the separation of the
mixture.
The flame will sterilize the water, so that water from the ocean will be
consumable. This is known as sanitation.
The flame will allow the pure water and salt water to separate into
different layers. This is known as density separation.
The flame will burn the salt component of the mixture, leaving only the
pure water behind for drinking. This is known as combustion.
The flame will allow the water to evaporate and be collected in its pure
form, leaving the salt component behind. This is known as distillation.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:20
Both 1,2−dihydronaphthalene and 1,4−dihydronaphthalene may be selectively hydrogenated to 1,2,3,4−tetrahydronaphthalene. one of these isomers has a heat of hydrogenation of 101 kj/mol (24.1 kcal/mol), and the heat of hydrogenation of the other is 113 kj/mol (27.1 kcal/mol). match the heat of hydrogenation with the appropriate dihydronaphthalene.
Answers: 2

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 04:00
What layer of the atmosphere is directly above the troposphere?
Answers: 1

You know the right answer?
A hypothetical situation of being stranded on a deserted island without water is often posed to stud...
Questions



Chemistry, 27.04.2021 19:10

Mathematics, 27.04.2021 19:10

Mathematics, 27.04.2021 19:10






Mathematics, 27.04.2021 19:10

Mathematics, 27.04.2021 19:10

Health, 27.04.2021 19:10

Biology, 27.04.2021 19:10


Mathematics, 27.04.2021 19:10


Mathematics, 27.04.2021 19:10

Mathematics, 27.04.2021 19:10

World Languages, 27.04.2021 19:10



