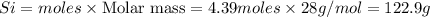The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
2Cr2O3(s)+3Si(s) > 4Cr(l)+3SiO2(s)
The reaction is begun with 161.00 g of Si and 139.00 g of Cr2O3.
How many grams of the excess reactant is left after the reaction is complete?

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 01:00
Water is important for the of cells. a: size, shape, and temperature b: temperature, color, and odor c: color, odor, and size d: shape, temperature, and color
Answers: 2

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3
You know the right answer?
The reaction of Cr2O3 with silicon metal at high temperatures will make chromium metal.
...
...
Questions



History, 02.12.2020 03:50


Mathematics, 02.12.2020 03:50

Chemistry, 02.12.2020 03:50


Mathematics, 02.12.2020 03:50


Advanced Placement (AP), 02.12.2020 03:50




Mathematics, 02.12.2020 03:50

Advanced Placement (AP), 02.12.2020 03:50

Mathematics, 02.12.2020 03:50


Mathematics, 02.12.2020 03:50

Mathematics, 02.12.2020 03:50

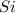 will be left from the given masses of both reactants.
will be left from the given masses of both reactants.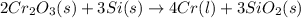
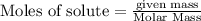
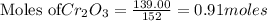
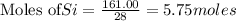
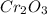 require 3 moles of
require 3 moles of 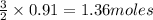 of
of