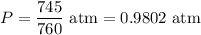Chemistry, 17.03.2020 06:35 Jonny13Diaz
Gas has a volume of 247.3 ML and is at 100 Celsius and 745 Hg. If the mass of the gas is 0.347 g what is the molar mass of the vapor?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Melting and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2

Chemistry, 23.06.2019 00:20
Steam reforming of methane ( ch4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. an industrial chemist studying this reaction fills a 1.5 l flask with 3.5 atm of methane gas and 1.3 atm of water vapor at 43.0°c. he then raises the temperature, and when the mixture has come to equilibrium measures the partial pressure of carbon monoxide gas to be 1 .0 atm. calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. round your answer to 2 significant digits.
Answers: 1

You know the right answer?
Gas has a volume of 247.3 ML and is at 100 Celsius and 745 Hg. If the mass of the gas is 0.347 g wha...
Questions

History, 03.07.2019 00:40

History, 03.07.2019 00:40





Physics, 03.07.2019 00:40



Physics, 03.07.2019 00:40

Physics, 03.07.2019 00:50

Chemistry, 03.07.2019 00:50


Physics, 03.07.2019 00:50

Mathematics, 03.07.2019 00:50


Business, 03.07.2019 00:50

History, 03.07.2019 00:50

History, 03.07.2019 00:50

Mathematics, 03.07.2019 00:50

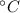 = 373 K
= 373 K