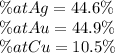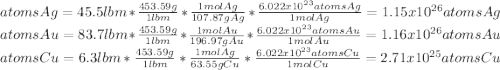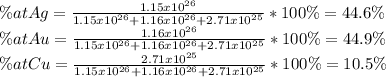Chemistry, 17.03.2020 06:08 elnkun98owvaa6
What is the composition, in atom percent, of an alloy that contains a) 45.5 lbm of silver, b) 83.7 lbm of gold, and c) 6.3 lbm of Cu? The atomic weights for silver, gold, and copper are, respectively, 107.87, 196.97, and 63.55 g/mol.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
What is the maximum number of electrons that an atomic orbital can contain?
Answers: 1

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1

Chemistry, 22.06.2019 11:00
An object becomes electrically charged when: electrons are created in it electrons from it are destroyed electrons are transferred to it protons from it are destroyed protons are created in it
Answers: 1

Chemistry, 22.06.2019 19:00
What information does a complete ionic equation give that the balanced equation doesn’t show?
Answers: 1
You know the right answer?
What is the composition, in atom percent, of an alloy that contains a) 45.5 lbm of silver, b) 83.7 l...
Questions

History, 19.09.2021 03:10



Mathematics, 19.09.2021 03:10

Mathematics, 19.09.2021 03:10


Chemistry, 19.09.2021 03:10


Chemistry, 19.09.2021 03:10

Mathematics, 19.09.2021 03:10



Physics, 19.09.2021 03:10

Mathematics, 19.09.2021 03:10

Mathematics, 19.09.2021 03:10

Mathematics, 19.09.2021 03:10

History, 19.09.2021 03:10

Spanish, 19.09.2021 03:10


English, 19.09.2021 03:10