
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: Cu2 (aq)+Zn () Cu (s)+Zn2(aq) Suppose the cell is prepared with 0.788 MCu2 in one half-cell and 7.32 M Zn2 in the other Calculate the cell voltage under these conditions. Round your answer to 3 significant digits. 2

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2
You know the right answer?
A galvanic cell at a temperature of 25.0°C is powered by the following redox reaction: Cu2 (aq)+Zn (...
Questions

Mathematics, 07.06.2021 16:20



Mathematics, 07.06.2021 16:20


English, 07.06.2021 16:20






Physics, 07.06.2021 16:20

English, 07.06.2021 16:20

Mathematics, 07.06.2021 16:20


Mathematics, 07.06.2021 16:20


Mathematics, 07.06.2021 16:20


Computers and Technology, 07.06.2021 16:20

 = -0.7618 V
= -0.7618 V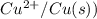 and anode is ().
and anode is ().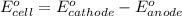
![E^{o} - (\frac{2.303 \times RT}{nF}) log {\frac{[Zn^{2+}]}^{1}{[Cu^{2+}]^{1}}](/tpl/images/0550/3152/96c78.png)
![E^{o} - (\frac{2.303 \times RT}{nF}) log {\frac{[Zn^{2+}]}^{1}{[Cu^{2+}]}^{1}](/tpl/images/0550/3152/d6ff2.png)
![E^{o} - (\frac{0.0591}{n}) log \frac{[Zn^{2+}]}^{1}{[Cu^{2+}]}^{1}](/tpl/images/0550/3152/6368f.png)



