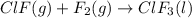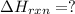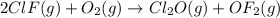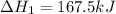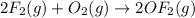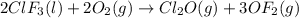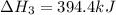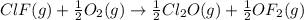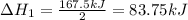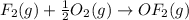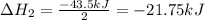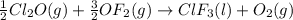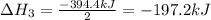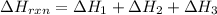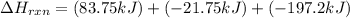Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an important fluorinating agent. Use the following thermochemical equations to calculate ΔH o rxn for the production of ClF3: (1) 2 ClF(g) + O2(g) → Cl2O(g) + OF2(g) ΔHo = 167.5 kJ (2) 2 F2(g) + O2(g) → 2 OF2(g) ΔHo = −43.5 kJ (3) 2 ClF3(l) + 2 O2(g) → Cl2O(g) + 3 OF2(g) ΔHo = 394.1 kJ

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:20
What is the ima of the 1 st class lever in the graphic given? 2 3 0.5
Answers: 1

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 08:00
Define dew point. i am writing this part to be able to ask the question
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
Enter your answer in the provided box. Oxidation of gaseous ClF by F2 yields liquid ClF3, an importa...
Questions



SAT, 24.04.2021 04:30

English, 24.04.2021 04:30

Mathematics, 24.04.2021 04:30


Mathematics, 24.04.2021 04:30


History, 24.04.2021 04:30



History, 24.04.2021 04:30


Mathematics, 24.04.2021 04:30


Mathematics, 24.04.2021 04:30


Mathematics, 24.04.2021 04:30


Arts, 24.04.2021 04:30

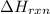 for the reaction is, -135.2 kJ
for the reaction is, -135.2 kJ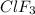 will be,
will be,