
Calculate the pH at the halfway point and at the equivalence point for each of the following titrations. 100.0 mL of 0.60 M titrated by 0.10 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.20 M pH at the halfway point = pH at the equivalence point = 100.0 mL of 0.70 M titrated by 0.15 M pH at the halfway point = pH at the equivalence point ='

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural.question 2 reflects a moral or social value.question 3 refers to something that can be measured.question 4 reflects a question that can’t be observed.
Answers: 1

Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2


Chemistry, 23.06.2019 00:30
What are the advantages of using the metric system? designed as a decimal system making conversions simpler more accurate system of measurement has prefixes that correspond to an amount to use with all base units used by the entire scientific community
Answers: 2
You know the right answer?
Calculate the pH at the halfway point and at the equivalence point for each of the following titrati...
Questions

Physics, 17.11.2020 20:00

English, 17.11.2020 20:00




Health, 17.11.2020 20:00

Mathematics, 17.11.2020 20:00






Chemistry, 17.11.2020 20:00

History, 17.11.2020 20:00

Biology, 17.11.2020 20:00

Arts, 17.11.2020 20:00




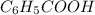 (
(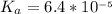 ) titrated by 0.10 M
) titrated by 0.10 M 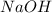
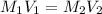 .
.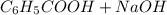 →
→ 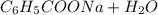
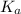 + log
+ log 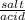
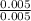 = 4.20
= 4.20

