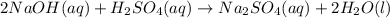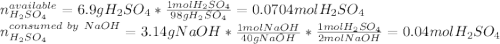Chemistry, 17.03.2020 04:36 GutsnGlory
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and liquid water . Suppose 6.9 g of sulfuric acid is mixed with 3.14 g of sodium hydroxide. Calculate the maximum mass of sodium sulfate that could be produced by the chemical reaction. Be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3


Chemistry, 23.06.2019 08:00
Which term means two or more atoms that share electrons in a chemical bond? a. hydrogen bond b. moleculec. ionic bondd. element amd you
Answers: 3
You know the right answer?
Aqueous sulfuric acid will react with solid sodium hydroxide to produce aqueous sodium sulfate and l...
Questions

SAT, 01.12.2020 01:00

English, 01.12.2020 01:00


English, 01.12.2020 01:00


Arts, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00

Arts, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00


Mathematics, 01.12.2020 01:00

Biology, 01.12.2020 01:00



Mathematics, 01.12.2020 01:00

Chemistry, 01.12.2020 01:00

Mathematics, 01.12.2020 01:00