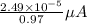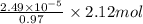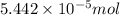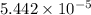Chemistry, 17.03.2020 04:40 hannahking1869
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon juice sample. A detector signal of 2.12 uA was observed. A standard addition of 1.00 mL of 24.9 mM ascorbic acid standard increased the current to 3.09 uA. Find the concentration of vitamin C in the watermelon juice.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 22.06.2019 05:50
Fill in the coefficients that will balance the following reaction: a0cr2(so4)3 + a1agno3 -> a2cr(no3)3 + a3ag2so4
Answers: 1

Chemistry, 22.06.2019 07:30
All cells are made of four types of acids, lipids, proteins, and carbohydrates.
Answers: 1

You know the right answer?
(a) [10 pts] Using an electrochemical method, you measured the ascorbic acid of a 30.0 mL watermelon...
Questions

Social Studies, 17.10.2019 19:00


Mathematics, 17.10.2019 19:00

Mathematics, 17.10.2019 19:00

Computers and Technology, 17.10.2019 19:00




Mathematics, 17.10.2019 19:00










Mathematics, 17.10.2019 19:00

History, 17.10.2019 19:00

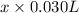
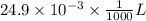
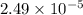 mole
mole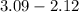

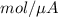 will be equal to as follows.
will be equal to as follows.