
Chemistry, 17.03.2020 04:05 lilrel8602
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different concentration of reactant was used during each trial, whereas the other factors were kept constant.
Which of the following statements explains which trial has a lower concentration of the reactant?
A. Trial 1, because the average rate of the reaction is lower.
B. Trial 1, because this reaction lasted for a longer duration than Trial 2.
C. Trial 2, because this reaction was initially fast and later slowed down.
D. Trial 2, because the volume of product formed per unit time was higher.
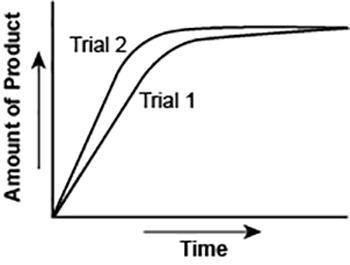

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 08:30
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 13:30
Mary is conducting an experiment on how pollution affects plant growth. how can she ensure that her data are reliable?
Answers: 3
You know the right answer?
The graph shows the volume of a gaseous product formed during two trials of a reaction. A different...
Questions

Social Studies, 06.05.2020 21:40


English, 06.05.2020 21:40





Mathematics, 06.05.2020 21:40




Advanced Placement (AP), 06.05.2020 21:40








Mathematics, 06.05.2020 21:40



