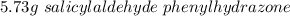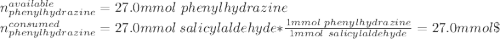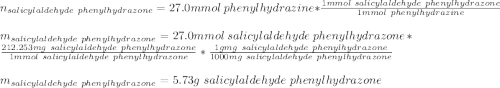Chemistry, 17.03.2020 03:14 michellelirett
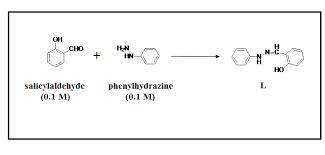
A 2.92 g (27.0 mmol) sample of phenylhydrazine was dissolved in 10 mL of 95% ethanol and stirred while 3.30 g (27.0 mmol) of salicylaldehyde, dissolved in 15 mL of ethanol, was added. what is the theoretical yield in grams for salicylaldehyde phenylhydrazone?

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Zinc + lead(ii) nitrate yield zinc nitrate + leadwhat's the chemical equation for this?
Answers: 1

Chemistry, 22.06.2019 00:40
Base your answer on the information below and on your knowledge of chemistry. nitrogen dioxide, no2, is a dark brown gas that is used to make nitric acid and to bleach flour. nitrogen dioxide has a boiling point of 294 k at 101.3 kpa. in a rigid cylinder with a movable piston, nitrogen dioxide can be in equilibrium with colorless dinitrogen tetroxide, n2o4. this equilibrium is represented by the equation below. 2no2(g) n2o4(g) + 58kj at standard pressure, compare the strength of intermolecular forces in no2(g) to the strength of intermolecular forces in n2(g).
Answers: 2

Chemistry, 22.06.2019 10:00
Part 1: include important facts found through your research. part 2: include your visual display. include your summary of “the chemistry of water” from the national science foundation website. include your experiment. part 3: include responses to the reflection questions.
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1
You know the right answer?
A 2.92 g (27.0 mmol) sample of phenylhydrazine was dissolved in 10 mL of 95% ethanol and stirred whi...
Questions

Mathematics, 16.06.2021 05:50

English, 16.06.2021 05:50



English, 16.06.2021 05:50

English, 16.06.2021 05:50

Biology, 16.06.2021 05:50

Mathematics, 16.06.2021 05:50

Spanish, 16.06.2021 05:50

Social Studies, 16.06.2021 05:50

Mathematics, 16.06.2021 05:50

Mathematics, 16.06.2021 05:50

History, 16.06.2021 05:50




Business, 16.06.2021 06:00



English, 16.06.2021 06:00