
Chemistry, 17.03.2020 02:23 miraclewhipppp
A 0.2722 g sample of a pure carbonate, X n CO 3 ( s ) , was dissolved in 50.0 mL of 0.1200 M HCl ( aq ) . The excess HCl ( aq ) was back titrated with 23.60 mL of 0.0980 M NaOH ( aq ) . How many moles of HCl react with the carbonate?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:30
Which mathematical relationship should you us to convert moles of a substance into grams
Answers: 1

Chemistry, 22.06.2019 03:40
Kc = 0.040 for the system below at 450oc. if a reaction is initiated with 0.40 mole of cl2 and 0.40 mole of pcl3 in a 2.0 liter container, what is the equilibrium concentration of cl2 in the same system? pcl5(g) ⇄ pcl3(g) + cl2(g)
Answers: 3

Chemistry, 22.06.2019 04:30
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 10:40
If an area has high air pressure and low humidity, what type of weather will it most likely have? plz !
Answers: 1
You know the right answer?
A 0.2722 g sample of a pure carbonate, X n CO 3 ( s ) , was dissolved in 50.0 mL of 0.1200 M HCl ( a...
Questions






Mathematics, 06.05.2020 05:31


Chemistry, 06.05.2020 05:31

Mathematics, 06.05.2020 05:31



Mathematics, 06.05.2020 05:32

Mathematics, 06.05.2020 05:32

Biology, 06.05.2020 05:32

History, 06.05.2020 05:32

Computers and Technology, 06.05.2020 05:32



Mathematics, 06.05.2020 05:32

Mathematics, 06.05.2020 05:32

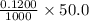 moles = 0.00600 moles
moles = 0.00600 moles
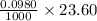 moles = 0.00231 moles
moles = 0.00231 moles

