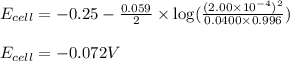Calculate the theoretical potential of the following cell. Indicate whether the reaction will proceed spontaneously in the direction considered (oxidation on the left, reduction on the right) or whether an external voltage source is needed to force this reaction to occur.
Pt, H2(757 torr)|HCl(2.00×10-4 M) parallel to Ni2+(0.0400 M)|Ni
the answer is
-0.072 V; External voltage needed
can anyone explain to me how i get this answer ?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:40
If the atomic mass of an atom is 34 and the atom contains 13 protons, how many neutrons does the atom contain?
Answers: 2

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 22:00
Does the number of ions in solution increase, decrease, or remain constant? it continuously decreases. it continuously increases. it decreases at first, then increases. it increases at first, then decreases.
Answers: 3

Chemistry, 23.06.2019 04:31
What are the coefficients that will balance the skeleton equation below? n2 + h2 → nh3
Answers: 1
You know the right answer?
Calculate the theoretical potential of the following cell. Indicate whether the reaction will procee...
Questions

Mathematics, 10.10.2019 19:00


Geography, 10.10.2019 19:00

Biology, 10.10.2019 19:00

Mathematics, 10.10.2019 19:00


Mathematics, 10.10.2019 19:00


Spanish, 10.10.2019 19:00


Biology, 10.10.2019 19:00


Mathematics, 10.10.2019 19:00

Mathematics, 10.10.2019 19:00

Mathematics, 10.10.2019 19:00

Biology, 10.10.2019 19:00


Mathematics, 10.10.2019 19:00


English, 10.10.2019 19:00

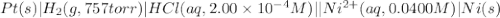
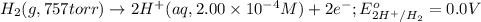
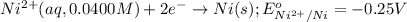
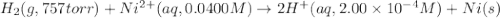
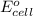 of the reaction, we use the equation:
of the reaction, we use the equation: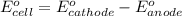
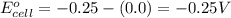
![E_{cell}=E^o_{cell}-\frac{0.059}{n}\log \frac{[H^{+}]^2}{[Ni^{2+}]\times p_{H_2}}](/tpl/images/0549/9866/bc143.png)
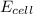 = electrode potential of the cell = ? V
= electrode potential of the cell = ? V![[H^{+}]=2.00\times 10^{-4}M](/tpl/images/0549/9866/69105.png)
![[Ni^{2+}]=0.0400M](/tpl/images/0549/9866/57673.png)
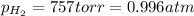 (Conversion factor: 1 atm = 760 torr)
(Conversion factor: 1 atm = 760 torr)