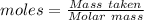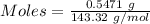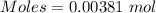Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 14:20
Calculate the enthalpy of the following reaction: 4 b (s) + 3 o2 (g) → 2 b2o3 (s) given the following pertinent information: (a) b2o3 (s) + 3 h2o (g) → 3 o2 (g) + b2h6 (g), δhoa = +2035 kj (b) 2 b (s) + 3 h2 (g) → b2h6 (g), δhob = +36 kj (c) h2 (g) + latex: \frac{1}{2} 1 2 o2 (g) → h2o (l), δhoc = −285 kj (d) h2o (l) → h2o (g), δhod = +44 kj
Answers: 3

Chemistry, 22.06.2019 02:30
The is a particle with one unit of positive charge a. proton b. positron c. electron d. nucleus awnser quick it is a important science test!
Answers: 2

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 12:00
Why are people not able to skip a dive to the deepest part of the ocean
Answers: 1
You know the right answer?
You have 0.2231 grams of an unknown monovalent salt (MCl). You treat it with an excess of AgNO3 and...
Questions

Health, 12.04.2021 06:10


Mathematics, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10




Arts, 12.04.2021 06:10





Mathematics, 12.04.2021 06:10




Mathematics, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10

Mathematics, 12.04.2021 06:10

Social Studies, 12.04.2021 06:10

 as:-
as:-