

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

Chemistry, 22.06.2019 09:30
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 14:30
Ahypothesis must be testable and falsifiable to be considered scientific a. trueb. false
Answers: 1

Chemistry, 22.06.2019 17:00
Which statement is true about a catalyst? a: a catalyst decreases the rate of the reaction. b. a catalyst is consumed during a chemical reaction. c. a catalyst lowers the activation energy of a reaction. d. a catalyst increases the reactant concentration during a reaction.
Answers: 1
You know the right answer?
Experimental Procedure, Part A.1. Calculate the mass of disodium (molar mass = 372.24 g/mol) requir...
Questions



Chemistry, 27.10.2021 17:30

Computers and Technology, 27.10.2021 17:30


Mathematics, 27.10.2021 17:30





Biology, 27.10.2021 17:30

Mathematics, 27.10.2021 17:30



Arts, 27.10.2021 17:30

English, 27.10.2021 17:30

Mathematics, 27.10.2021 17:30

Mathematics, 27.10.2021 17:30

Medicine, 27.10.2021 17:30

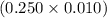 moles = 0.0025 moles
moles = 0.0025 moles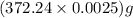 = 0.93 g
= 0.93 g

