

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
In apex! a liquid heated beyond a certain temperature becomes
Answers: 1

Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1


Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
A 1.00 L flask is filled with 1.15 g of argon at 25 ∘C. A sample of ethane vapor is added to the sam...
Questions




Biology, 23.06.2021 19:30


Biology, 23.06.2021 19:30






English, 23.06.2021 19:30

Mathematics, 23.06.2021 19:30


Mathematics, 23.06.2021 19:30



Physics, 23.06.2021 19:30

Mathematics, 23.06.2021 19:30

Mathematics, 23.06.2021 19:30

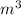
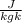
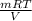
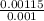 × 208.13 × 298
× 208.13 × 298 

