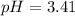Chemistry, 17.03.2020 00:57 claytonhopkins
A 1.50 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after the addition of 0.100 moles of solid NaOH. Assume no volume change upon the addition of base. The Ka for HF is 6.8 × 10-4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1

Chemistry, 22.06.2019 22:30
Which is a characteristic of the electron sea model for metallic bonding? molecular orbitals overlap to produce bands. electrons flow easily between metal nuclei. electrons are in fixed positions in the orbitals. atomic nuclei are arranged in an irregular pattern.
Answers: 3

Chemistry, 23.06.2019 03:30
Name 3 types of energy you see being used as you look around a classroom
Answers: 1
You know the right answer?
A 1.50 L buffer solution is 0.250 M in HF and 0.250 M in NaF. Calculate the pH of the solution after...
Questions




Geography, 27.05.2021 03:50





Mathematics, 27.05.2021 03:50




Mathematics, 27.05.2021 03:50


Mathematics, 27.05.2021 03:50

Mathematics, 27.05.2021 03:50

English, 27.05.2021 03:50



History, 27.05.2021 03:50

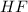 .
.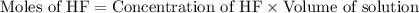
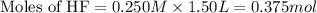
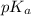 .
.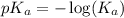
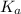 in this expression, we get:
in this expression, we get: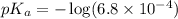
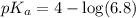
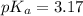
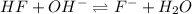
![pH=pK_a+\log \frac{[Salt]}{[Acid]}](/tpl/images/0549/7799/e961a.png)
![pH=pK_a+\log \frac{[F^-]}{[HF]}](/tpl/images/0549/7799/bef2f.png)
![pH=3.17+\log [\frac{(\frac{0.475}{1.50})}{(\frac{0.275}{1.50})}]](/tpl/images/0549/7799/a4ae7.png)